THE FORMATION OF NEVADA TURQUOISE
02/07/2016

In this photo of Stone Mountain Turquoise you should note the very light green almost white color at the bottom of the stone. This is an excellent example of the unique varieties of Nevada Stone Mountain Turquoise. This example demonstrates Zinc being introduced into the Gem Stone matrix.
Nevada Turquoise is comprised of the chemical elements copper (Cu), Aluminum (Al), phosphorus (PO4) and water (H2O). The mineral description is described as a Hydrated Aluminum Copper Phosphate. The actual nomenclature of the Mineral in all three states is:
TURQUOISE – Chemical Formula: CuAl6 (PO4)4 (OH)8 + 4H2O
FAUSTITE – Chemical Formula: (Zn, Cu) Al6 (PO4)4(OH)8 · 4H2O
CHALCOSIDERITE – Chemical Formula: Cu (Fe+3, Al) 6 (PO4)4(OH) 8 · 4H2O
The two (2) additional elements Zn (Zinc) and Fe (Iron) when incorporated into the molecular structure of Turquoise will and does influence the color and hardness of the Turquoise.
Nevada Turquoise is formed when the priority minerals are present in the exact proportions and are subjugated to the required physical and chemical exchange processes. Existing donor minerals that are present in surface area rocks are reduced and dissolved into a liquid matrix and deposited in cracks, and hollows that have been created in a host rock. These solutions of liquid matrix saturated solutions will continue to add and build for periods of time in excess of several million years or more.
Nevada Turquoise can be fractured; the maximum hardness is just under 6 which is a little harder than window glass. Characteristically Turquoise is a cryptocrystalline mineral and in rare instances it does form in single crystal; however this is an extreme rarity (See solidified quartz photo included in the Supergene – Hypogene sectional report). The properties of Nevada Turquoise are variable to a degree. The crystal system is triclinic, the Specific Gravity will range from 2.60 g/cm3 to 2.91g/cm3, however, the Specific Gravity can be affected by the cryptocrystalline grain size. The more compact the higher the Specific Gravity and conversely the less compact the lower the Specific Gravity. The fracture of Turquoise is irregular and uneven, considered sub-conchoidal. The cleavage is perfect and the Tenacity is Brittle, the Luster is Sub-Vitreous, Waxy or Earthy. It is normally opaque; however it can be Translucent and even Transparent. The Streak is Pale greenish blue to white and is considered insoluble in anything other than heated Hydrochloric Acid. The Refractive Index via Sodium light at 589.3 nm is approximately 1.61 – 1.62 utilizing a gemological refractometer and is a direct result of the polycrystalline nature of Turquoise. Under longwave UV light Turquoise may fluoresce Green, Yellow, or bright Blue. There is no reaction to UV light short wave.
THE FORMATION OF NEVADA TURQUOISE
SUPERGENE and HYPOGENE
There are two avenues of deposition for of matrix material to be introduced into the host rock. The first was normally accepted as the only way the Turquoise laden feed solutions could form Turquoise in the host rock. The name SUPERGENE was applied to this process by the EXPERTS. The total depth of the Supergene application was a total of 20 meters or 66 feet. It became necessary to explain how and why Turquoise was being discovered and mined at the 60 meters or 200 foot depths. These facts shot holes in the EXPERTS hypotheses that Turquoise could only be formed at depths to 66 feet. One of the draw backs to this hypothesis is the fact that the beginning elevations were never considered when the Turquoise was first formed. If you consider the total time frame of 2 to 3 million years, a lot of geological changes can and have come about.
The name of the additional avenue that the EXPERTS hung on this process is HYPOGENE . This process furnishes matrix feed stock via deep avenues of quartz intrusions into the host rock. When the Quartz intrusion begins to cool, crystals of Turquoise and other minerals begin to form. Now one of the draw backs to this avenue is the hardness of the Turquoise. The Turquoise and other minerals will be soft with a very low Hardness value. But as time goes on these veins of Turquoise will harden, remember the time frame of 2 to 3 million years. A lot of heat can be generated, just from the depths that are involved. Not to mention all of volcanic activity going on.
This is an example of a solidified liquid Quartz intrusion:

So when you think about that natural Nevada Turquoise in that pendant or that ring or those earrings you are wearing just think about the millions and millions of years that it took to create your special pretty and remember it was created by the hand of the ultimate artist.
THE FORMATION CHEMISTRY OF NEVADA TURQUOISE
(BLUE & GREEN TURQUOISE)
02/13/2016
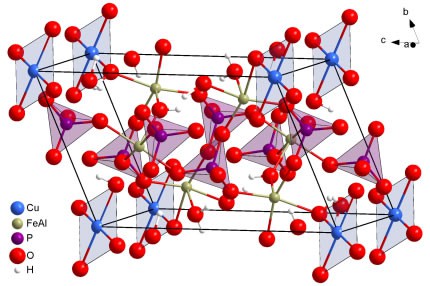
This is a 3 Dimensional representation of a molecule of Copper – Iron – Turquoise
Nevada Turquoise Chemistry formation is a product of its Geology. The basic building block minerals must be present in the native Geology to form Turquoise. This supposition I am sure may seem to be elementary in logic, however the processes required to extract and reform the components of Turquoise in the exact proportions are not straight forward.
BLUE TURQUOISE

BLUE Turquoise is comprised of the chemical elements copper (Cu), Aluminum (Al), phosphorus (PO4) and water (H2O). The mineral description is described as a Hydrated Aluminum Copper Phosphate. The following elements are required: Copper (Cu2), Aluminum (Al2), Phosphorus (PO4) and Water (H2O).
BLUE Turquoise can only exist as Cu Al6 (PO4)4(OH)8 · 4(H20). The percent (%) weights of each element that must be present to produce BLUE Turquoise are:
|
ELEMENT REQUIRED |
PERCENT |
COMPOUND FORMED |
|
Aluminum |
37.60% |
Al2O3 |
|
Copper |
9.78% |
CuO |
|
Phosphorus |
34.90% |
P2O5 |
|
Water |
17.98% |
H2O |
|
100.00% |

GREEN Turquoise is comprised of the chemical elements copper (Cu), Iron (Fe+3), Aluminum (Al), phosphorus (PO4) and water (H2O). The mineral description is described as a Hydrated Aluminum Copper Phosphate. The following elements are required: Copper (Cu2), Iron (Fe+3), Aluminum (Al2), Phosphorus (PO4) and Water (H2O).
GREEN Turquoise can only exist as Cu (Fe+3 Al6) (PO4)4(OH)8 · 4(H20). The percent (%) weights of each element that must be present to produce GREEN Turquoise are:
|
ELEMENT REQUIRED |
PERCENT |
COMPOUND FORMED |
|
Aluminum |
36.51% |
Al2O3 |
|
Copper |
9.06% |
CuO |
|
Iron |
0.22% |
Fe2O3 |
|
Phosphorus |
34.10% |
P2O5 |
|
Water |
20.11% |
H2O |
|
100.00% |
The elements required must be available in the geological formation. There are no other outside components with the exception of water. Here is a short list of some geological donors that make this batch of cookies possible. Additionally this list only includes the donor elements specific to the formation of the Turquoise, the list DOES NOT include matrix rock or inclusions that ARE NOT Turquoise. This will be addressed later in Geological Formations.
NOTE: This list is comprised of donor compounds that are SPECIFIC to Nevada Turquoise. You SHOULD NOT automatically INFER that all Turquoise is formed utilizing the same donor compounds. Each Geological profile is different and must be evaluated based on the specific Geological conditions that exist for a specific Turquoise. The only caveat to this rule is the actual Turquoise mineral that has been formed. The chemical formula of Turquoise will not change regardless of the state or country that it hales from. It is best that you know where your Turquoise comes from.
|
REQUIRED ELEMENT |
DONOR SOURCE |
REQUIRED ELEMENT |
DONOR SOURCE |
|
Phosphoric Acid (P2O5) |
Carbonate Fluorapatite |
Copper (CuO) |
Chalcopyrite |
|
Tricalcium Phosphate |
Chrysocolla |
||
|
Aluminum (Al2O3) |
Feldspars |
Iron (Fe2O3) |
Pyrite |
|
Plagioclase |
Limonite |
||
|
Anorthite |
Hematite |
||
|
Water (H2O) |
Rain Water |
ABOUT THE AUTHOR
William (Bill) Ebbs Jr. is from San Antonio, Texas and made his presence known to the world July 4, 1945. He has a multifaceted background to include Department Store Manager and graduating from the United States Infantry College at Fort Benning, Georgia. At present He is retired from the work force per se, however he is and has been the owner and operator of a small exotic gem business since 2005. He was prior to retirement an Environmental Professional working for a worldwide agrichemical company. He has extensive background in writing and the execution of Analytical protocols for Chemistry, Geology, Meteorology, Biology and Quality Assurance – Quality Control. He has been published in co-authored papers by Battelle International and is the holder of several corporate patents.


Comment from Facebook | Bill Machovina: Do you know if there’s a minimum % of copper required in order for it to be considered turquoise?